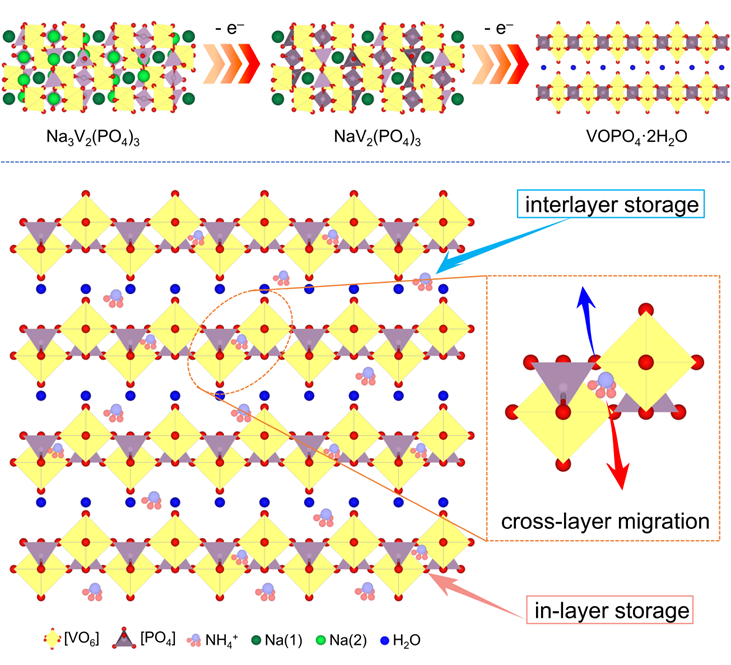
Non-metallic cations, such as NH4+, have demonstrated rapid kinetic transmission and superior rate capabilities when used as carriers in aqueous secondary batteries. The small hydrated ion size (3.31 Å) and its unique topological chemical properties indicate its vast potential for applications. To date, numerous studies have focused on ammonium ion storage materials, with transition metal compounds having tunnel and layered structures drawing significant attention. However, the majority of the research on layered materials predominantly emphasizes interlayer hydrogen bond diffusion mechanisms, often overlooking the functionality of the intrinsic layer structure. In actuality, channels also exist on the layer itself, analogous to tunnels within the layers of materials. This suggests that ammonium ions can not only migrate between layers but also undergo cross-layer transfer within them. Consequently, it is of paramount importance to re-evaluate the role of these in-layer channels in the storage and migration processes of ammonium ions.
In light of this, Assistant Professor Zhuoxin Liu of Shenzhen University, in collaboration with Professor Chunyi Zhi of City University of Hong Kong and Assistant Professor Hongfei Li of Southern University of Science and Technology, employed electrochemical phase transition techniques to synthesize layered VOPO4·2H2O (E-VOPO) primarily grown on the (200) crystal face. The research demonstrated that the tetragonal channels on the (001) layer not only serve as storage sites for ammonium ions but also enhance ionic migration kinetics by providing rapid in-layer pathways. Compared to the conventional VOPO4·2H2O (VOPO), the synthesized E-VOPO showcased superior ammonium ion storage capabilities, boasting a significantly increased specific capacity, remarkable rate performance, and reliable cycling stability. By pairing the E-VOPO anode with a CuFe Prussian blue analog (CuFe PBA) cathode, the constructed full cell consistently operated through 12,500 charge-discharge cycles at 2A g−1, running continuously for over 70 days. These findings, titled "Unlocking High-Performance Ammonium-Ion Batteries: Activation of In-Layer Channels for Enhanced Ion Storage and Migration", were published in the prestigious journal Advanced Materials. The first author of this article is Dr. Xiangyong Zhang, a postdoctoral researcher at Shenzhen University.
Full article page:https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202304209
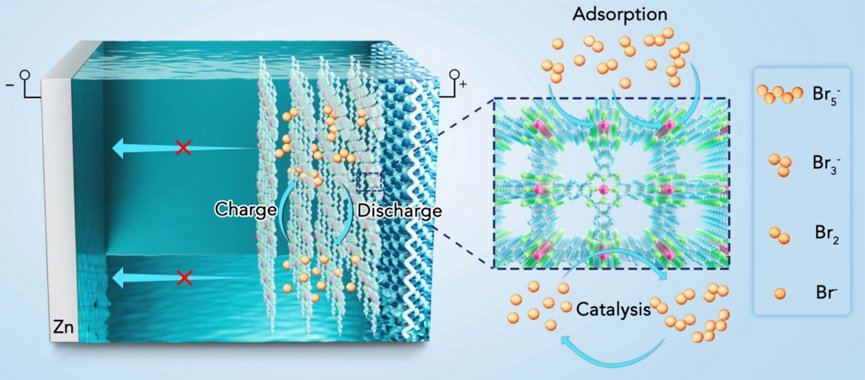
Aqueous non-flow zinc-bromine batteries have shown tremendous potential for energy storage systems due to their low cost, high safety, and high capacity. However, the conversion-type bromine cathode faces significant challenges, including capacity loss from the formation of polybromides and their shuttling effect, and slow reaction kinetics due to bromine's insulating nature. Previous research has primarily focused on developing cathode hosts with large surface areas, strong adsorption capabilities, and high conductivity to address these issues. Nonetheless, the limited adsorption/hosting sites and persistent diffusion of polybromides pose considerable challenges for the practical application of non-flow zinc-bromine batteries. Furthermore, the discharge voltage of reported aqueous zinc-bromine batteries typically reaches ≤1.75 V, which is notably less than the theoretical value of 1.84 V.
To address the aforementioned issues, Assistant Professor Zhuoxin Liu of Shenzhen University, Professor Chunyi Zhi of City University of Hong Kong, and Assistant Professor Hongfei Li of Southern University of Science and Technology developed a high-performance aqueous NF-ZBB that employs a two-dimensional conjugated metal-phthalocyanine nickel (NiPPc) as an adsorption-catalysis cathode host. The NiPPc cathode host presents several distinct advantages: (1) Atomically dispersed Ni-N4 sites offer a plethora of catalytically active sites. Compared to carbon atoms, these dense metal centers exhibit relatively stronger polarity, providing rich adsorption sites for bromine and polybromide ions. (2) The π-π stacked layered phthalocyanine-based metal-organic framework ensures effective charge carrier transport, thereby enhancing redox kinetics. (3) The porous structure favors high bromine loading. As a result, the non-flow zinc-bromine batteries constructed using the NiPPc host witnessed remarkable improvements in electrochemical performance, encompassing specific capacity, rate capability, cycle stability, and discharge plateau voltage. Employing potassium bromide as the active material, the battery demonstrated a high specific capacity (240 mAh g−1KBr) at a current density of 5 A g−1, maintaining approximately 95% capacity retention and about 99% Coulombic efficiency over 3000 cycles. NiPPc's significant catalytic activity also enabled the battery to achieve a high discharge plateau voltage of up to 1.82 V at a current density of 2 A g−1, which is close to the theoretical discharge voltage. These findings, titled "Boosting aqueous non-flow zinc–bromine batteries with a two-dimensional metal–organic framework host: an adsorption-catalysis approach", were published in the prestigious journal Energy & Environmental Science. The first author of this article is Dr. Hua Wei, a postdoctoral researcher at Shenzhen University.

Full article page:https://doi.org/10.1039/D3EE01639K