With the gradual depletion of global fossil energy and the increasingly severe environmental problems, technologies for the acquisition of green hydrogen (green ammonia) energy and the clean conversion and utilization of waste have attracted great attention from countries around the world. A large amount of nitrate and aldehyde (e.g., formaldehyde) wastes and pollutants are generated during the global industrialization and agriculturalization processes, posing significant hazards to human health and ecosystems. Due to the high energy consumption, long time consumption, and high cost of current purification technologies, their applications still face enormous challenges.
Rational design of cathode and anode reactions is expected to significantly reduce the energy consumption of hydrogen (ammonia) production. The concept of the nitrate reduction-formaldehyde oxidation (NO3RR–FOR) electrochemical system has only begun to attract attention very recently. Based on the thermodynamic principles of electrode reactions, it is easy to find that the theoretical potential of nitrate reduction to ammonia (φƟ = +0.69 V, vs. RHE, the same as follows) is more positive than that of aldehyde (formaldehyde φƟ = −0.22 V) oxidation for hydrogen production, and the raw materials for both electrode reactions are derived from industrial and agricultural wastes. Theoretically, this system is highly attractive: the combination of them forms a "formaldehyde-nitrate" galvanic cell system for power generation, with a theoretical discharge window of 0.91 V, and at the same time, it should be able to obtain high-value products such as green hydrogen and green ammonia.
However, nitrate reduction to ammonia undergoes an eight-electron process, with complex reaction pathways and many possible side reactions (possible by-products such as nitrite and nitrogen), resulting in a very high kinetic activation energy barrier and thus a high overpotential. Formaldehyde oxidation also has different electron pathways, among which the reaction conditions are harsh for one-electron oxidation to produce hydrogen, and the selectivity is difficult to control. To realize ammonia production at the cathode and aldehyde oxidation for hydrogen production at the anode while generating electricity, several important challenges still exist: (1) How to accurately realize the regulation of H* exchange behavior; (2) How to optimize the reaction pathway to achieve high catalytic selectivity; (3) The insufficient stability of the catalyst itself. Although there have been very few previous studies exploring the coupled system of nitrate reduction (NO3RR) and formaldehyde oxidation (FOR), they are still limited by key bottlenecks such as no hydrogen generation, inability to output electrical energy, and excessive reliance on easily deactivated copper-based catalysts. It remains very challenging to realize the integration of "hydrogen production, ammonia production, and power generation" using copper-free catalysts.
Recently, Associate Professor Zhao Bin from the research group of Academician Luo Jing-Li, College of Materials Science and Engineering of Shenzhen University, proposed a Ni-Pd driven "formaldehyde-nitrate" four-in-one electrochemical system. The relevant results were published in J. Am. Chem. Soc., with Zhao Bin and Professor Luo Jingli as the corresponding authors.
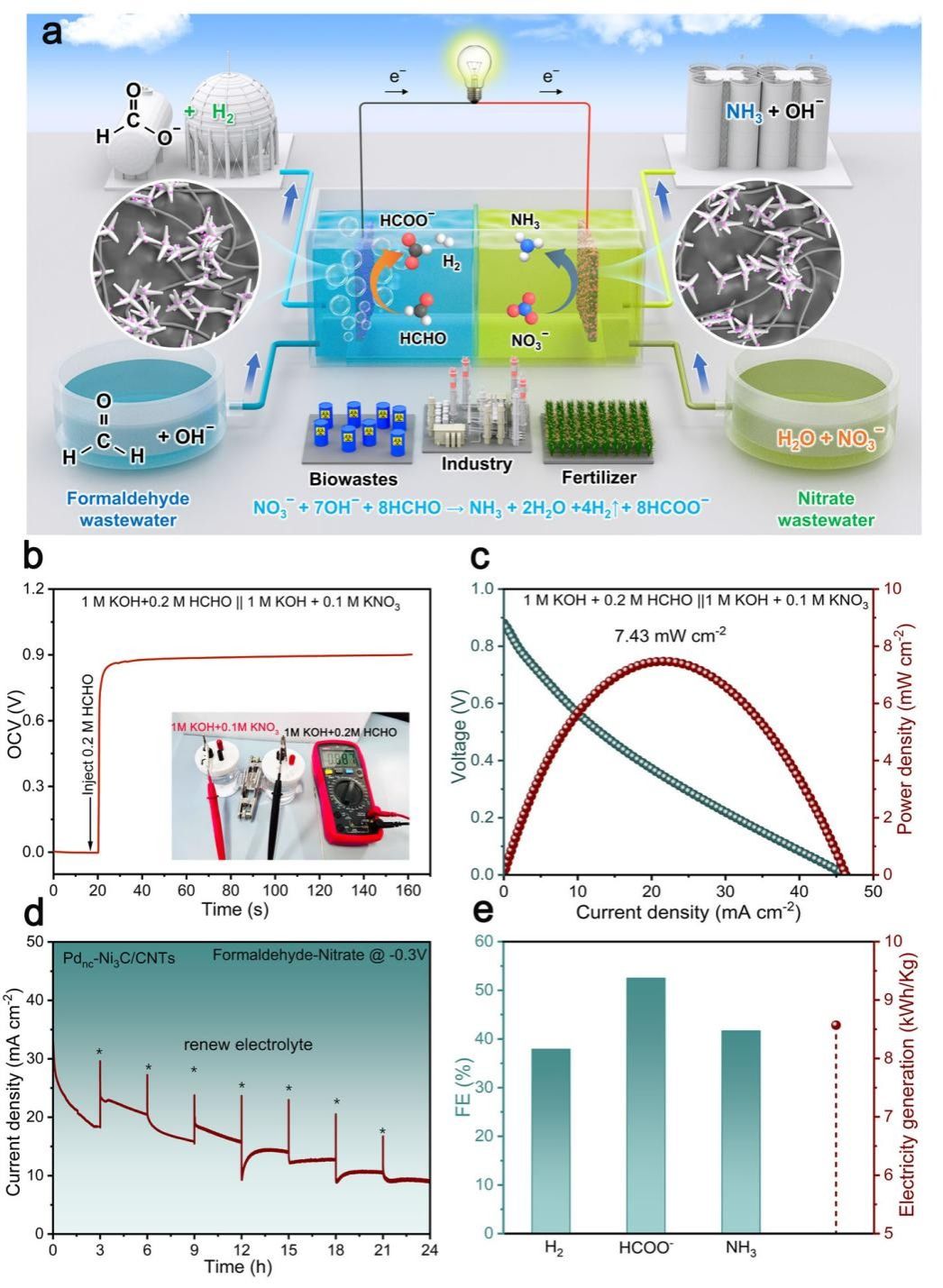
Fig. (a) Schematic diagram of the design of the "formaldehyde-nitrate" four-in-one electrochemical energy system, which can synchronously convert wastes into multiple non-mixed high-value-added products (ammonia, H2, formate) through value-added reactions and generate electrical energy. (b) Open-circuit voltage of the "formaldehyde-nitrate" system. (c) Discharge curve and power density change curve. (d) Discharge stability at 0.3 V for 24 hours. (e) Faradaic efficiencies of simultaneous production of H2, ammonia, and formate in the formaldehyde-nitrate catalytic power generation coupled system, and calculation of synchronous power generation.
This work proposes a copper-free bifunctional catalyst—palladium nanoclusters supported on nickel carbide (Ni3C) (Pdnc-Ni3C). Relying on its excellent H* exchange capability, this catalyst accurately regulates H adsorption and transfer behavior, realizing bidirectional catalysis of NO3RR and FOR. The above H* utilization and transfer mechanism was characterized in depth using isotope-labeled in-situ spectroscopic methods. On the cathodic NO3RR side, Pdnc-Ni3C exhibits an onset potential of +0.27 V vs. RHE and achieves a Faradaic efficiency of 98% for ammonia production at −0.3 V vs. RHE; on the anodic side, the onset potential of FOR is as low as 0.04 V vs. RHE, with a wide oxidation window of 0 ~ 1.2 V vs. RHE and a high current density of up to 910 mA cm−2, whose performance is significantly superior to previously reported copper-based and nickel-based systems. This should be the first report of nickel-based catalysts for low-potential selective formaldehyde oxidation. Among them, the synergistic effect between Ni3C and Pd clusters significantly improves the current density and efficiently promotes rapid hydrogen production under the one-electron transfer pathway. Differential electrochemical mass spectrometry (DEMS) first reveals the "intermolecular coupling pathway" of selective oxidation and hydrogen evolution of formaldehyde, deepening the understanding of the one-electron formaldehyde oxidation mechanism. By coupling NO3RR and FOR, a high-performance "formaldehyde-nitrate" galvanic cell with an open-circuit voltage of 0.88 V and a peak power density of 7.4 mW cm−2 was successfully constructed. Particularly importantly, this work uses nickel-based catalysts (instead of copper-based catalysts) to successfully drive the "FOR-NO3RR" cell to achieve "four-in-one" multi-functional integration: while generating electricity, it can convert wastes into H2, NH3, and formate, demonstrating a strategy of synergy between chemical value addition and energy generation.
Paper Information:
https://pubs.acs.org/doi/10.1021/jacs.5c11608
Modulating Hydrogen Exchange Capabilities by Heterogenizing Pd Nanoclusters onto Ni3C Multipods for Efficiently Driving the “Formaldehyde-Nitrate” Tandem Electrochemical System
Zulakha Zafar, Bin Zhao*, Rida Javed, Arunpandiyan Surulinathan, Xin Long, M. Bilal Hussain, Ning Chen, Renfei Feng, Yu Zhang, Xian-Zhu Fu, Jing-Li Luo*
J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c11608
The first completing affiliation of this paper is Shenzhen University, and Associate Professor Zhao Bin is the first corresponding author. This work was supported by the National Natural Science Foundation of China (General Program), Guangdong Basic and Applied Basic Research Foundation, and Shenzhen Science and Technology Program.
Editor: Zhang Xiaoying
Typesetting:
Reviewers: Wang Dong , Wang Lei
